There are three different contexts in which one can view any given neurotransmitter: as a chemical which interacts with other chemicals in certain ways, as a ligand which binds to certain receptors in order to produce certain cellular effects, and as a modulator which affects cellular (generally neuronal) function in certain ways. Hence, we'll have three stories — the chemical, cytological, and neuroscientific – which, while nearly independent of one another, all shed light on the various disorders, processing pathways, and psychotropics that rely on the neurotransmitter.
Note: Basically none of the graphics in this article will be original; most of them will probably be taken from Wikipedia.
Note 2: I have basically zero formal education in chemistry or biology, and there is no advice to be found here; if you mistakenly read any actionable suggestions into my mere descriptions, don't blame me if you spontaneously combust.
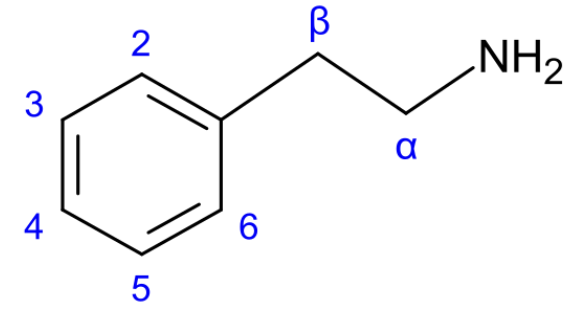
Almost all chemicals we're going to talk about in the dopamine section descend from the chemical phenethylamine, abbreviated PEA. If atoms are the letters of the language of organic chemistry, then functional groups are the syllables, and phenethylamine is trisyllabic:
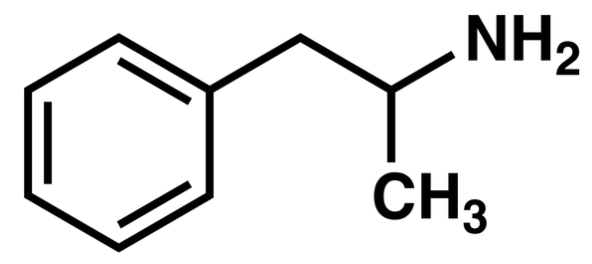
It is typical to label the carbons, as this makes it easier to label molecules which are formed by adding groups to phenethylamine, of which we'll encounter a bunch; the canonical numbering of PEA's carbons is shown above. For instance, $\alpha$-methylphenethylamine is the molecule given by adding a methyl group, $CH_3$, to the $\alpha$ carbon of PEA.
This molecule is generally abbreviated: alpha-methyl phenethylamine is the full name of amphetamine, the main ingredient in Adderall. If we add a methyl group to the nitrogen, an N-methyl, then we get methamphetamine. (A lot of stimulating drugs are phenethylamines).
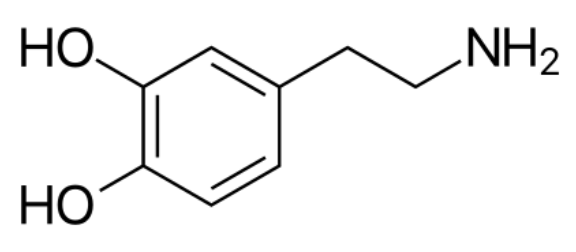
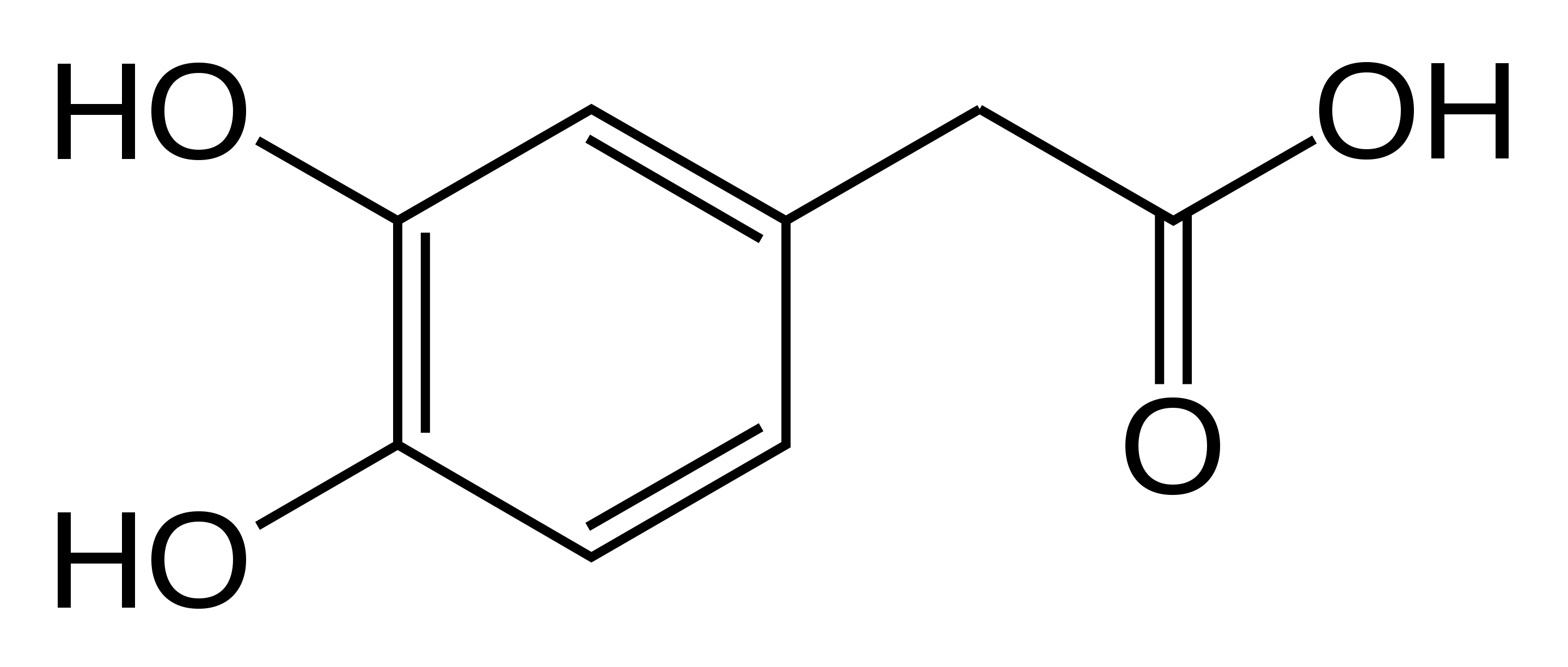
In this naming system, dopamine is 3,4-dihydroxyphenethylamine: it has two hydroxyl groups, HO-, attached to its 3 and 4 carbons, giving the molecule on the right. While dopamine can be viewed as a phenethylamine derivative, it can also be viewed as a catecholamine: this is a kind of molecule which has on one end a catechol group, or a hexagon with two hydroxyl groups side-by-side, and which also has a nitrogen, making it an amine. Both points of view — dopamine as phenethylamine and dopamine as catecholamine — will be useful in their own right.

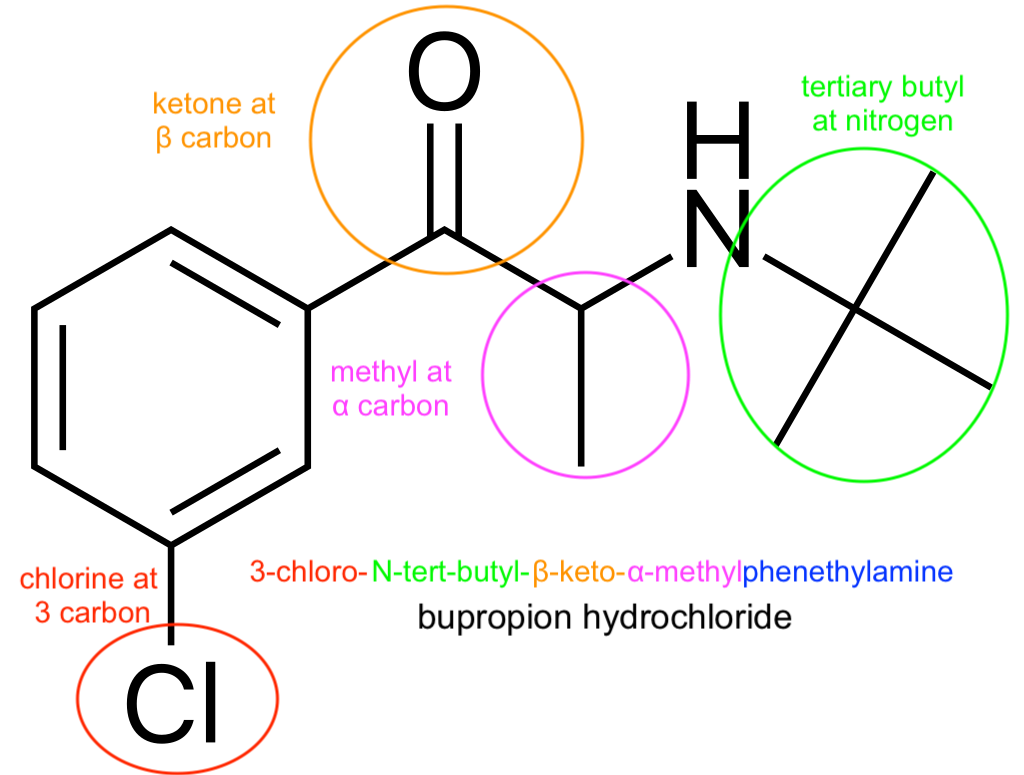
For fun, to the right is the derivation of the chemical name of bupropion, the phenethylamine-based antidepressant. (There's a standardized scheme for determining the order of the prefixes).
Since there's plenty of space here, I may as well name some other phenethylamines:
As dopamine cannot cross the blood-brain barrier, what dopamine is present in the brain is produced in the brain. It is synthesized from amino acids through multiple different pathways; these pathways are actually very simple, when considered in terms of functional groups.
An amino acid is a molecule which consists of an amine group $NH_2$ connected to a carboxyl group $COOH$ by a central carbon, the $\alpha$-carbon; of the two additional bonds this $\alpha$-carbon can form, one is a hydrogen, but the other varies, with each amino acid having its own unique side chain. The orientation of the hydrogen and side chain can vary, making most amino acids chiral, with mirror image levo- (L) and dextro- (D) forms. We shall generally deal with the L-forms of amino acids.
Phenylalanine, for instance, has side chain given by a benzyl group, or a ring with an extra carbon. Tyrosine is almost exactly like phenylalanine, but has an extra hydroxyl (OH) group at the end of its side chain. These amino acids are both aromatic: they have very stable rings. (Apparently, the first few compounds of this family smelled pleasant, so they were called aromatic; it was discovered that they had a stable ring in common, and the name was taken to refer to stable ring-containing compounds more generally).
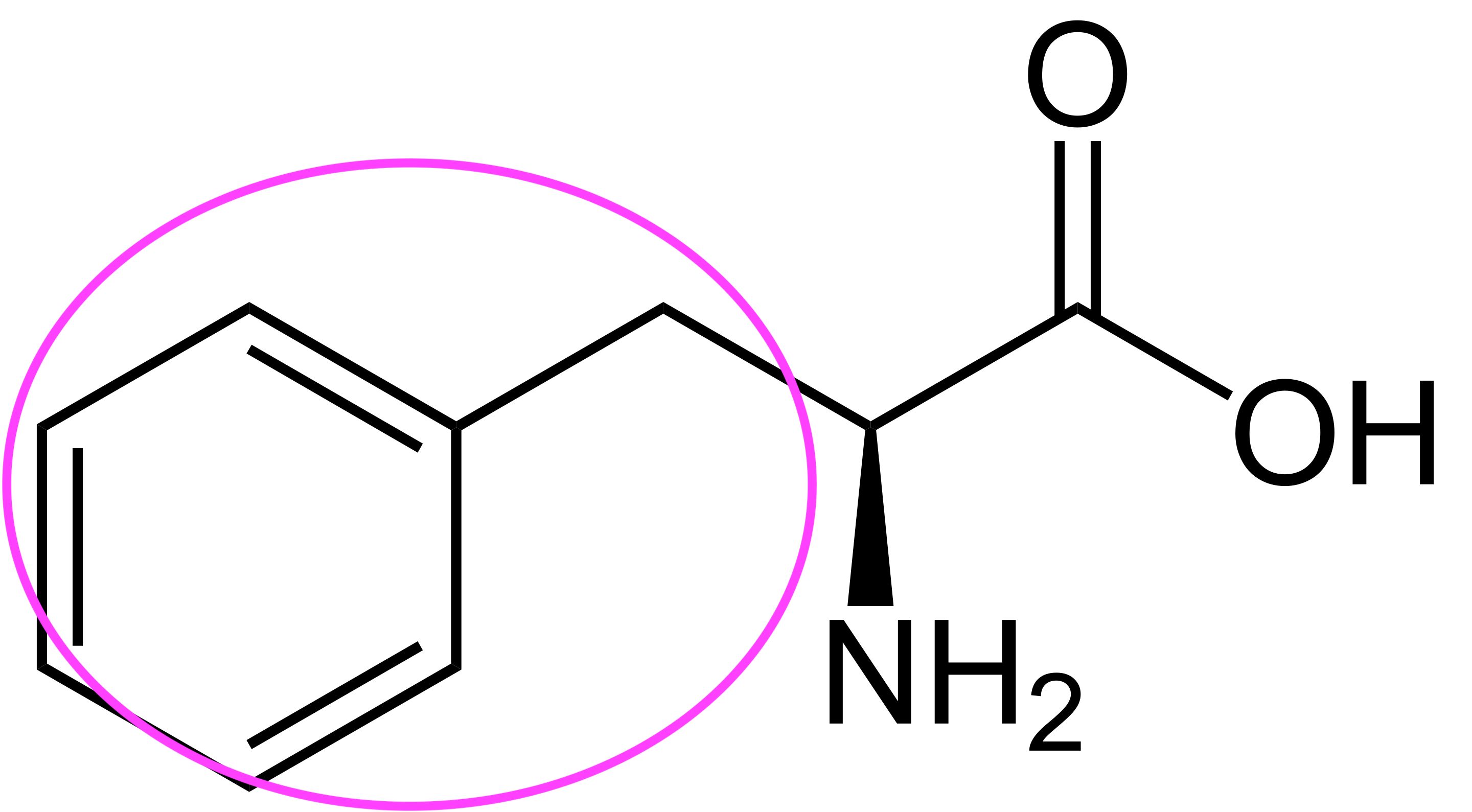
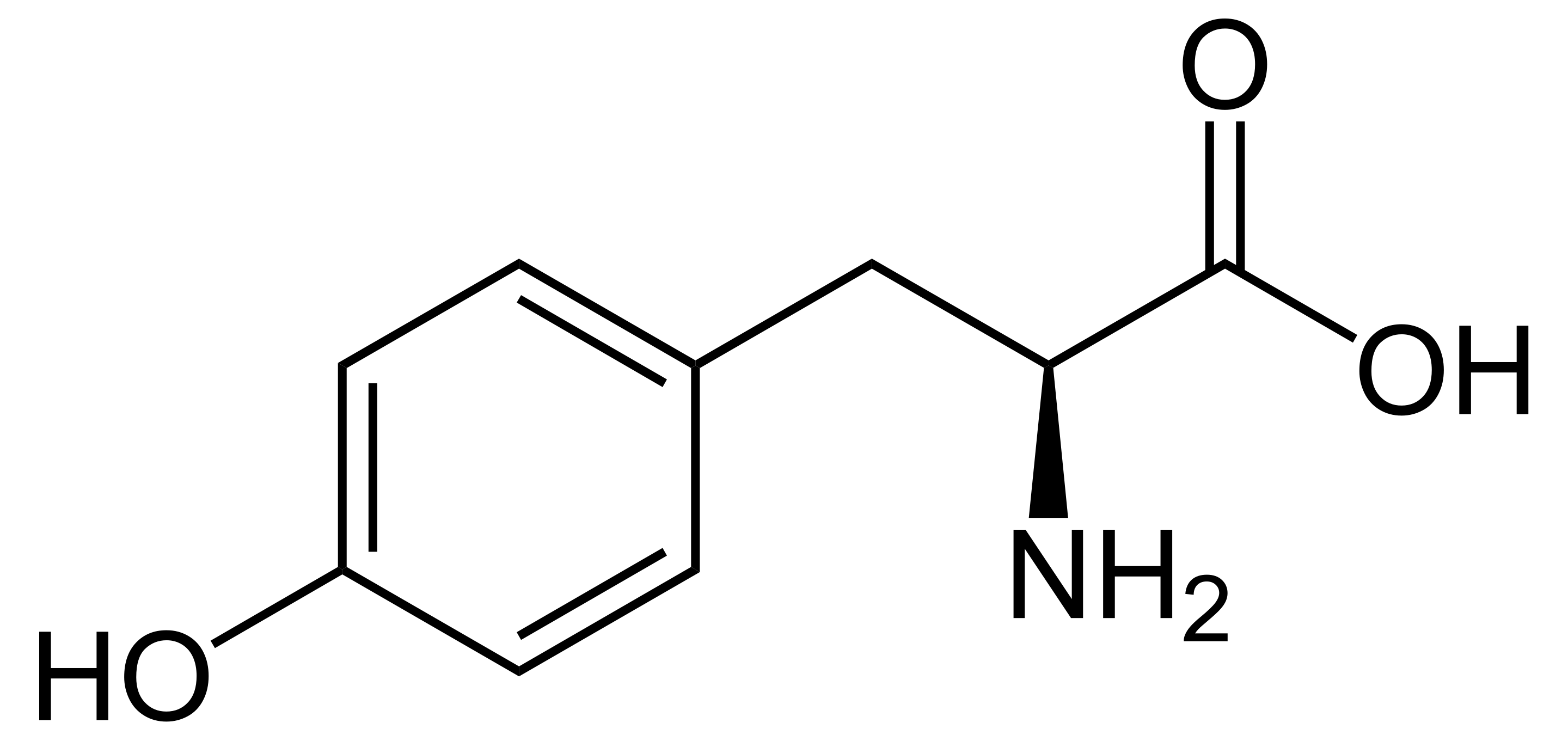
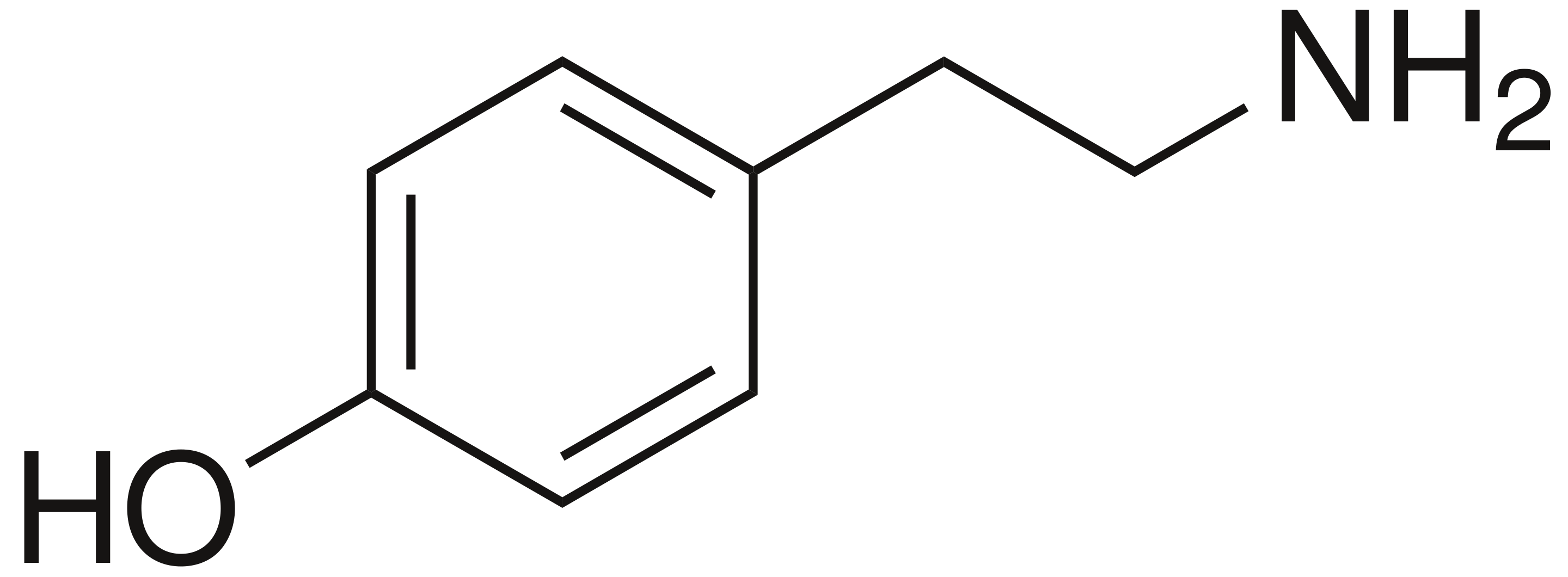
Note the structural resemblance of phenylalanine to phenethylamine: all we need to do is clip off the carboxyl group! The enzyme that removes the carboxyl from phenylalanine removes the carboxyl from all aromatic amino acids, so long as they're in their L-form; it's conveniently known as aromatic L-amino acid decarboxylase, aka AADC. AADC turns L-phenylalanine directly into phenethylamine, and turns L-tyrosine into a molecule known as tyramine; this is simply 4-hydroxyphenethylamine.
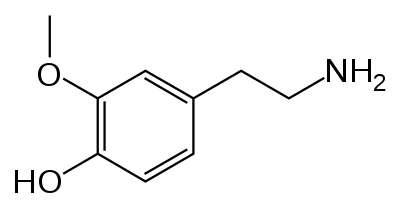
Note: "Tyramine" is often called p-tyramine; the p stands for para-, which is a way of saying that the hydroxyl is added to the carbon opposite the rest of the atom. If it is added to one of the carbons directly next to the rest of the atom (the 2' or 6' carbons), we say ortho-, and if it is added to one of the in-between carbons (3' or 5'), it is known as meta-. Hence, tyramine = p-tyramine = 4-tyramine = 4-hydroxyphenethylamine, m-tyramine is 3-hydroxyphenethylamine, and o-tyramine is 2-hydroxyphenethylamine. The same goes for tyrosine: the form of tyrosine above is p-tyrosine, but we might also put the hydroxyl group on the 3' carbon and call it m-tyrosine. Note that AADC will turn normal tyrosine into p-tyramine, but will turn m-tyrosine into m-tyramine.
At the same time that this is going on, there is a family of enzymes that add hydroxyl groups to aromatic amino acids: members of this family are known as aromatic amino acid hydroxylases (AAAH). The AAAH phenylalanine hydroxylase does exactly what it says on the tin: by adding a hydroxyl group to (usually the 4-)carbon of phenylalanine (phenylalanine and tyrosine have the same carbon numbering as phenethylamine), it turns phenylalanine into tyrosine. Tyrosine 3-hydroxylase then adds a second hydroxyl group to the 3-carbon, turning it into a new molecule known as L-DOPA. (L-DOPA is technically an amino acid, though it doesn't seem to be considered as such).
.svg.png)
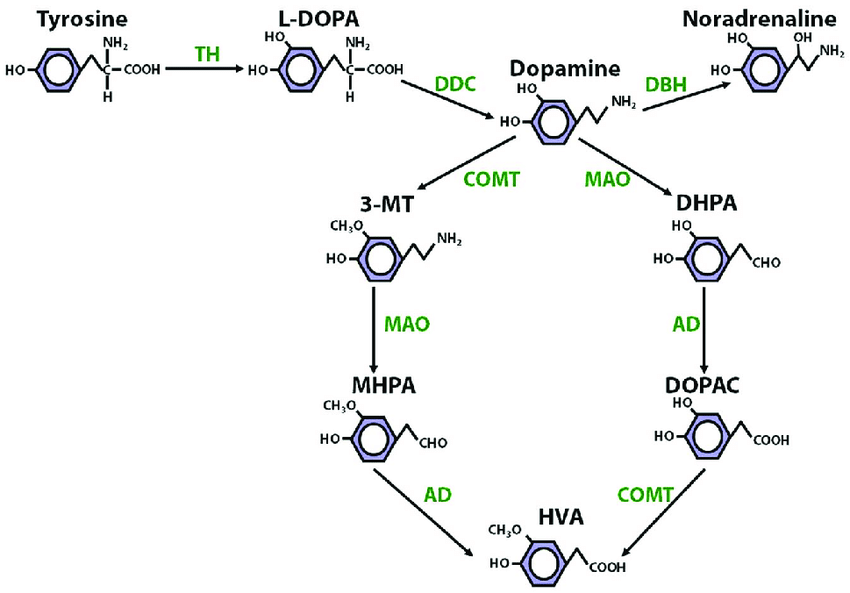
There are three different routes to producing dopamine:
The first pathway is the most common method of dopamine synthesis, with the others making minor contributions. Hence, the rate-limiting step in the production of dopamine occurs in the first pathway, particularly in the production of L-DOPA from L-tyrosine by tyrosine hydroxylase. Both L-DOPA and L-tyrosine can cross the blood-brain barrier, so oral administration of either of them is capable of increasing dopamine in the brain -- but the latter's rate-limitation by TH makes it preferable to the former, as completely bypassing the rate-limiting step may change the distribution of dopamine throughout the brain, potentially leading to severe side-effects.
All dopamine is eventually metabolized into homovanillic acid, in which the 4' hydroxyl group is replaced with a methoxyl ($H_3CO$) group, and the amine group is replaced with a carboxyl group. This happens through two parallel pathways.
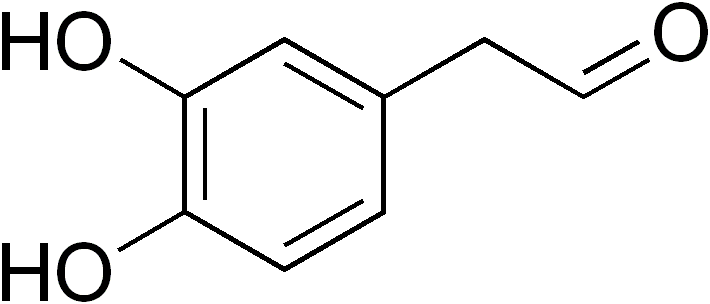
In the first pathway, the monoamine oxidase (MAO) family of enzymes work to replace the amines in monoamine neurotransmitters (a group which we will get to shortly, which includes dopamine) with aldehyde ($C = O$) groups. The enzyme MAO-A acts on many different monoamines, while MAO-B acts primarily on dopamine. After they act on dopamine, it becomes 3,4-dihydroxyphenylacetaldehyde (DOPAL).
Aldehyde dehydrogenase (ALDH) is an enzyme which replaces aldehydes in many different molecules with carboxyl groups; it acts on DOPAL to produce 3,4-dihydroxyphenylacetic acid (DOPAC). Finally, catechol-O-methyltransferase adds a methyl group to the 4' oxygen, turning DOPAC into homovanillic acid (HVA). To summarize, this pathway is $\text{Dopamine} \overset{\text{MAO}}{\longrightarrow} \text{DOPAL} \overset{\text{ALDH}}{\longrightarrow}\text{DOPAC} \overset{\text{COMT}}{\longrightarrow} \text{HVA}$. HVA has no known biological function, though it's commonly measured as a proxy for dopamine levels.
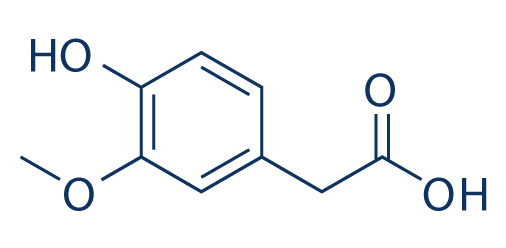
The second pathway utilizes the same enzymes in a different order: COMT comes first, turning dopamine into 3-methoxytyramine, which is then turned into 3-methoxy-4-hydroxyphenylacetic acid by ALDH, and then into HVA by MAO.
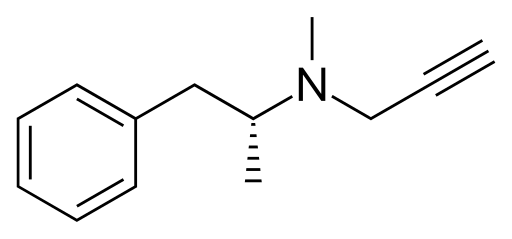
A common psychiatric strategy to increase dopamine levels is to inhibit the action of MAO, which slows down the degradation of dopamine; one molecule that does this is selegiline, which is formed by adding a methyl and propynyl ($-C-C\equiv C$) group to the nitrogen atom of L-amphetamine.
There's only one other enzyme that turns dopamine into something else, so we may as well mention it: dopamine $\beta$-hydroxylase (DBH) is an enzyme that acts on dopamine by, unsurprisingly, hydroxylating the $\beta$ carbon. This makes it into norepinephrine.
To summarize this section:
Now we move up a level, abstracting away the actual chemical reactions. If dopamine is to affect brain function, it must do so by affecting the behavior of actual cells, and it does this by binding to cell receptors so as to modify their behavior. All the receptors that dopamine binds to are G protein-coupled receptors (GPCRs), so it'll be useful to discuss those in themselves.
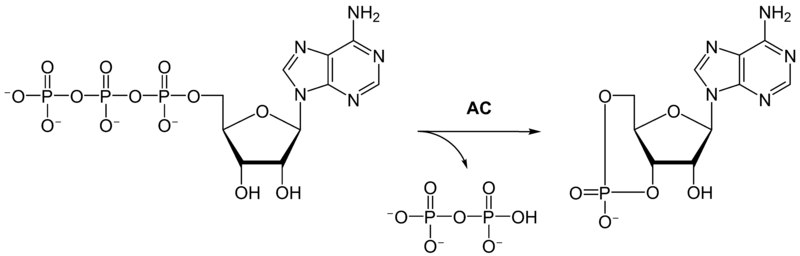
G proteins are essentially switches: they normally bind to guanosine diphosphate (GDP), but when an extra phosphate is added to GDP, making it into guanosine triphosphate (GTP), the G protein switches "on" and undergoes a conformational change.
There are many pathways that activated $G_\alpha$ subunits trigger:
The enzymes protein kinase A (PKA) and protein kinase C (PKC) have an activity proportional to cAMP and calcium ion levels, respectively; these protein kinases modify the behavior of other proteins by phosphorylating them. This is one way in which G protein signaling cascades end up actually doing things, as we'll see later.
G protein-coupled receptors (GPCRs) are a family of membrane receptors which also act as switches. When a ligand binds to a GPCR outside of the cell, it activates the receptor (causes the receptor to undergo a conformational change), and this activated receptor can, on the inside of the membrane, turn the GDPs of specific kinds of G proteins into GTPs, switching them on.
Up- and down-regulation of dopamine reception happens primarily on this level of abstraction, namely as a specific case of a mechanism common to G protein-coupled receptors in general. G protein-coupled receptor kinases (GRKs), whose activity is dynamically regulated by GPCR activity both directly and indirectly through many mechanisms, phosphorylate GPCRs; this has the dual effect of (a) causing the internalization of receptors and (b) allowing certain proteins known as arrestins to bind to receptors, blocking them. (In humans, there are exactly four arrestins; two modulate photoreceptors and two (the $\beta$-arrestins) modulate neurotransmission). Other regulatory mechanisms act on top of this to modulate the rate at which receptor proteins are transcribed and translated from their genes.
G protein-coupled receptors are often called metabotropic receptors. These form one of the two categories of receptors employed by the nervous system; the other consists of the ionotropic receptors, so named because they mediate the transmission of ions across the cell membrane. Of relevance to us are two specific families of ionotropic receptors: the AMPA receptors and NMDA receptors. (The names are abbreviations of certain agonists of each of these receptors, which don't really matter). Both of these require glutamate in order to open,
There are two kinds of proteins which transport dopamine:
There are three families of receptors to which dopamine binds:
First, the individual trace-amine associated receptor TAAR1, which is an intracellular receptor; this is coupled to the G proteins $G_s$ and $G_q$, as well as to some GIRKs. $G_{s\alpha}$ and $G_{q\alpha}$ activate adenylyl cyclase and PLC, respectively, so TAAR1 activation increases both cAMP and cytosolic calcium concentration; through some mechanism I can't find information on, TAAR1 activation also has the potential to activate GIRKs, hyperpolarizing the cell.
Anyway, the punchline: when the internal receptor TAAR1 is activated by dopamine, it increases both cAMP and cytosolic calcium concentration, thereby activating PKA and PKC; these kinases phosphorylate DAT, causing it to shuttle dopamine out of the cell. That TAAR1 activation can also hyperpolarize cells prevents neurons which are excited by external dopamine from becoming hyperactive as a result of all the extra dopamine being pumped out of the cell. All in all, then, TAAR1 agonists cause dopamine to leave the cell and enter the extracellular matrix, where it can bind to other, external receptors.
Second, the "D1-like" dopamine receptors D1 and D5; these are coupled to $G_s$, and thereby increase cAMP levels.
Third, the "D2-like" dopamine receptors D2, D3, and D4; these are coupled to $G_i$, whose subunit $G_{i\alpha}$ inhibits adenylyl cyclase, thereby decreasing cAMP levels.
The functions of cAMP, and therefore the D1-like and D2-like receptors, seem to differ markedly from neuron to neuron. This makes it difficult to speak of the function of the D family of receptors without resorting to vagaries, but this paper, which everyone seems to link to, has some information
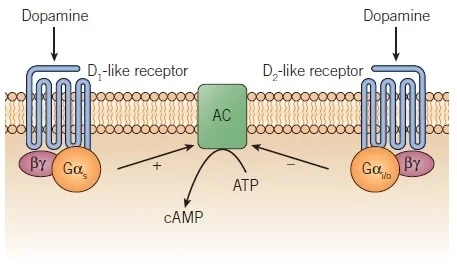
Dopamine can also modulate plasticity: the increase/decrease in cAMP caused by dopamine reception sometimes triggers intracellular changes that increase or decrease expression of the ionotropic receptors that neurons use to communicate with one another, and can also activate proteins that block or unblock existing receptors. (See here for a detailed review).
This is enough context to explain precisely how some drugs act on dopamine transmission:
Of course, these drugs, while primarily acting on dopamine, also act on other neurotransmitters such as serotonin and norepinephrine.
We know what dopamine is—how it's structured, produced, and used by cells—but what does it actually do in the brain? What's the point of its presence in the brain? Here, we cover how dopamine modulates neural activity. This is, while the most important thing to discuss, also the most difficult. Chemistry, proteins, intracellular messaging pathways—all of these are not only very well-known to science, but structured in relatively systematic ways that admit broad overviews. Neither of these seem to be the case with the neuromodulatory properties of dopamine, so this is where things get messy.
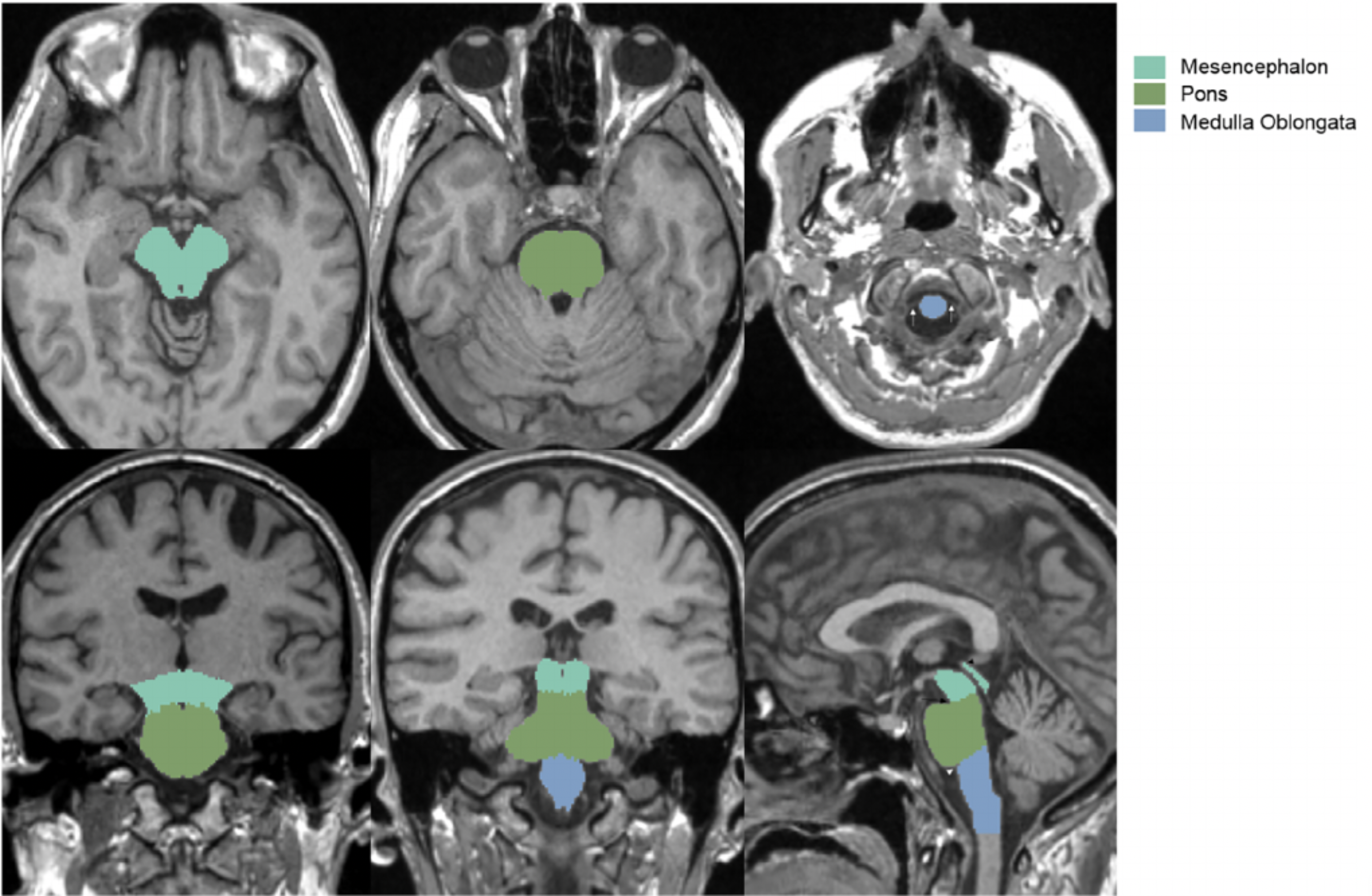
The midbrain is a small portion of the brain located at the very top of the brainstem (see image to the right).
From an evolutionary perspective, it is one of the oldest parts of the brain, and is present in almost all vertebrates. Naturally, it handles extremely low-level functions. Unfortunately for us, it is also very complex, with many different small and distinct parts. It is traditionally split into two levels, each of which I've provided below.
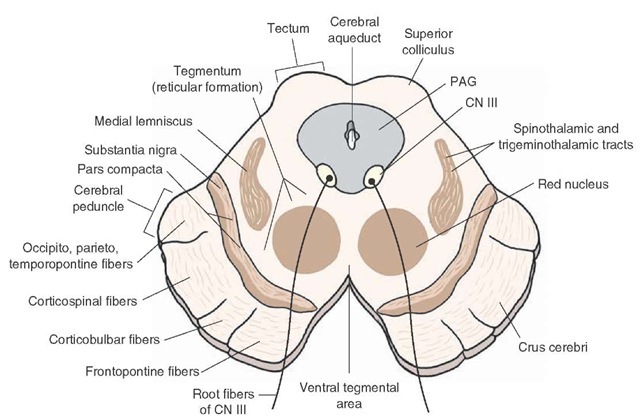
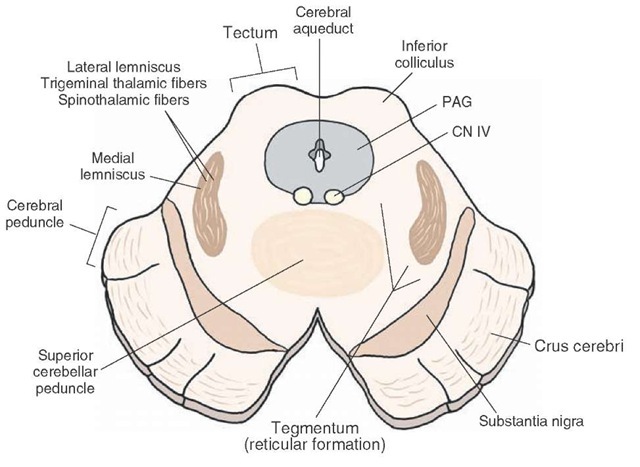
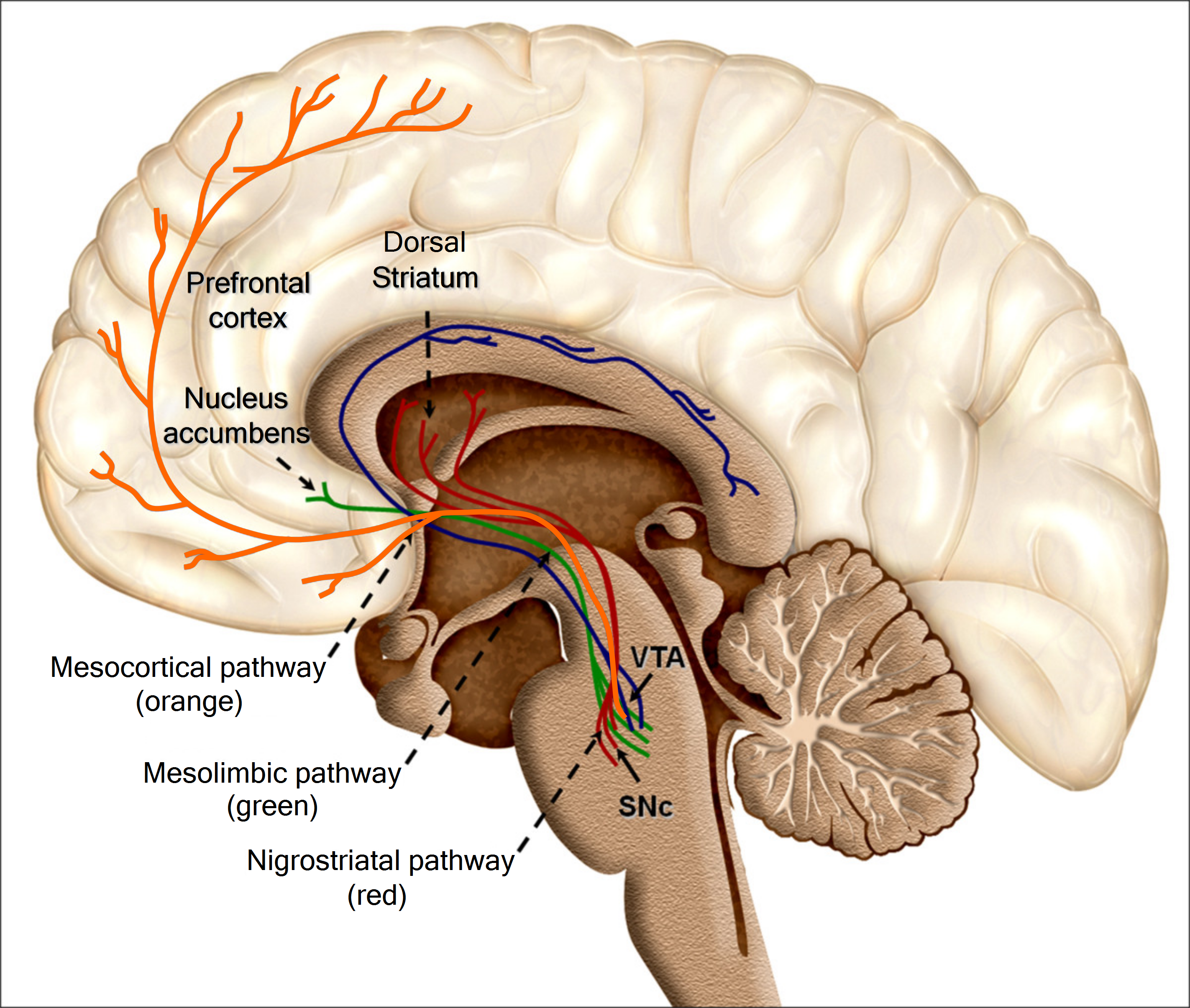
Out of the approximately 100 billion neurons in the human brain, only around 400,000 produce dopamine; these dopaminergic neurons form a small number of clusters spread throughout the brain. It isn't enough to simply profile each of these clusters, as they project to many other regions of the brain, over which they have great control; we need to profile these regions as well. However, profiling the clusters is a start. They have a standard numbering, originally given by Dahlström and Fuxe (1964) and extended as new regions were found: there are groups A8 through A17, Aaq, and the telencephalic DA group.
.svg.png)
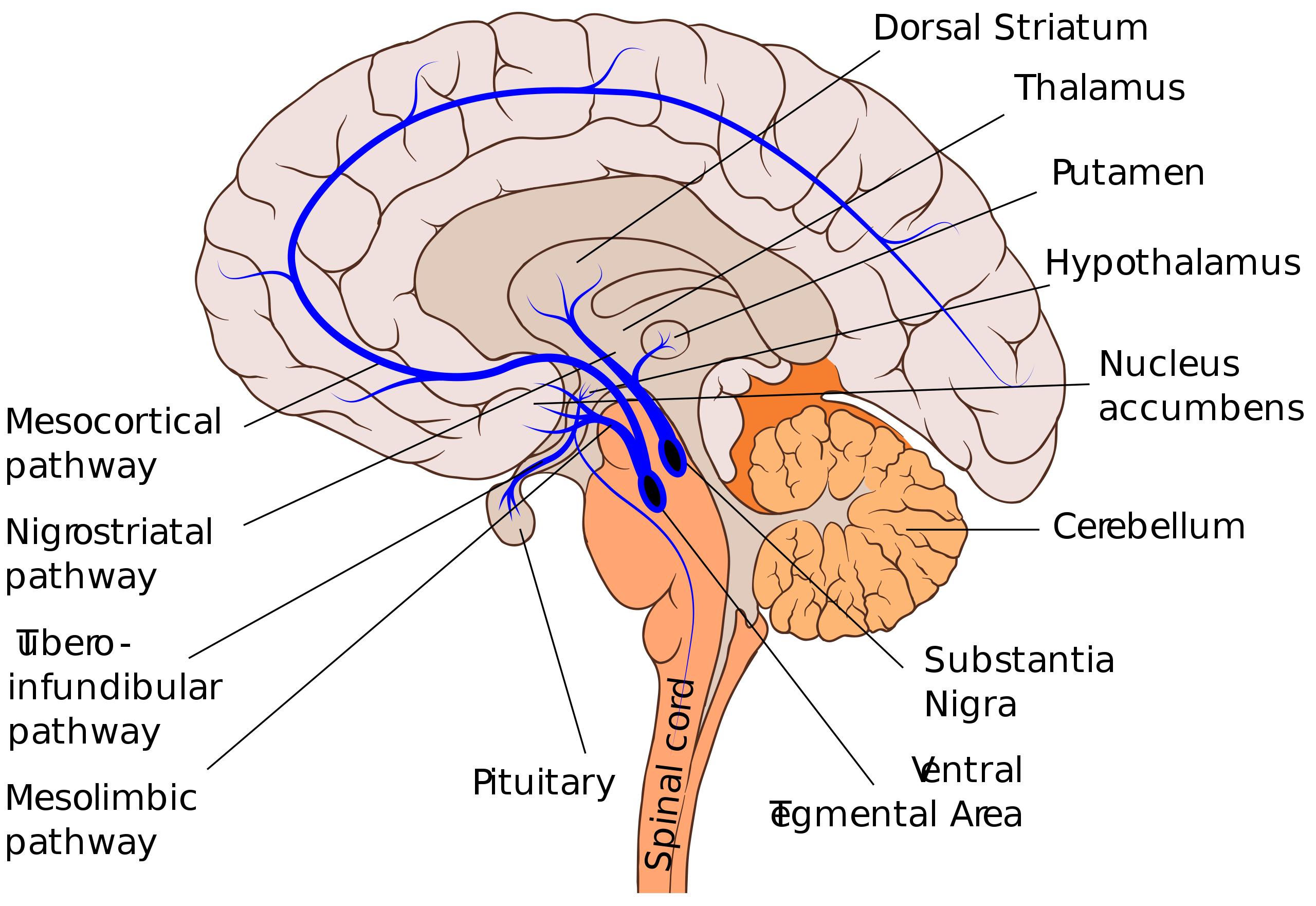
Let's restrict our focus to dopaminergic groups A9 (in this section) and A10 (in the next section), and the pathways coming from them, i.e. the mesolimbic, mesocortical, and nigrostriatal pathways. Here, we'll finally start to explain the actual effects of dopamine on brain function. As stated, dopaminergic group A9 is located in the substantia nigra pars compacta (SNc), and projects primarily to the dorsal striatum, i.e. the putamen and caudate nucleus. It also has minor projections to the cortex and ventral striatum. This is the what — but how does the SNc use dopamine to modulate the behavior of the striatum?
At any given moment, the brain sees many possible opportunities to move — different stimuli, goals, obstacles, and so on. For instance, if I'm reading something and hear a noise outside, I might continue to move my eyes down the page, or I might move them towards the source of the noise. The striatum is involved in deciding which action to take. Naturally, this is divided into two parts:
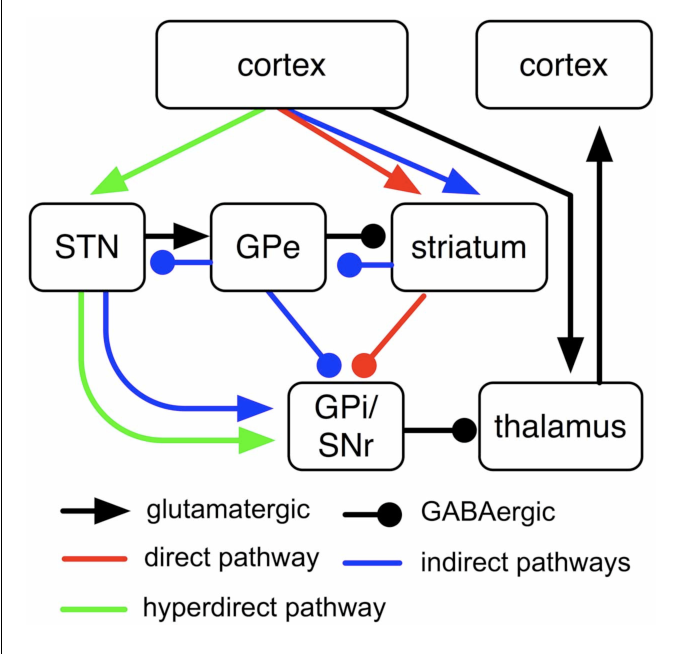
Thus, via this pair of parallel loops, together known as the cortico-basal ganglia-thalamo-cortical (CBGTC) loop, the dorsal striatum is capable of affecting motor cortex activity towards certain ends. How does dopamine modulate the dorsal striatum? Those neurons on the dorsal striatum that lie on the direct pathway tend to express the dopamine receptor D1, while those on the indirect pathway tend to express D2. In both populations, intracellular cAMP levels correspond to excitability; hence, as D1 activation increases cAMP levels, dopamine increases excitability on the direct pathway, and as D2 activation decreases cAMP levels, dopamine decreases excitability on the indirect pathway. (Source 1, 2).
Example: Parkinson's disease is characterized by the death of A9 dopaminergic neurons. The cause of this cell death is unknown, but it results in the depletion of dopamine in the nigrostriatal pathway. As above, this decreases the excitability of the direct pathway, while increasing the excitability of the direct pathway. This results in the characteristic motor symptoms of Parkinson's — difficulty starting movement, due to an overactive indirect pathway, and difficulty controlling movement, due to an underactive direct pathway. L-DOPA administration can, by replenishing this dopamine supply, alleviate these symptoms, though long-term L-DOPA use causes dyskinesia after several years (in patients who already have Parkinson's, source).
A wealth of experiments support the hypothesis that dopamine also modulates reinforcement learning in the striatum (source). Specificially, when an action results in an unexpected reward, the substantia nigra dopaminergic neurons that seem to respond to that reward are excited, and when an action fails to result in an expected reward, they are inhibited; when an action results in the reward (or lack thereof) expected, the neurons fire at their normal rate (source).
Both the mesolimbic and mesocortical pathways originate from group A10 in the ventral tegmental area (VTA), located in the midbrain. Their combination is known as the mesocorticolimbic projection, AKA the reward system. This is what most people think of when they think of dopamine, though we have much less knowledge about the mechanisms by which dopamine works here than we do about the mechanisms by which it works in the nigrostriatal pathway.
The mesocortical pathway, which projects to the prefrontal cortex, is by far more obscure than the mesolimbic pathway. Not much about the rat's mesocortical pathway can be generalized to humans, due to the massive prefrontal cortex differences (whereas the midbrain is relatively conserved). Hence, while we have some idea of the "what" — executive functions — we seem to have very little idea of the "how".
While the nigrostriatal pathway projects to the dorsal striatum, the mesolimbic pathway projects to the ventral striatum
The story of acetylcholine isn’t as simple as that of dopamine — it doesn’t fit into a family of molecules varying in a very modular way, as do the phenethylamines with their neatly varying carboxylations and hydroxylations. Actually, it occurs as the intersection of two other stories:
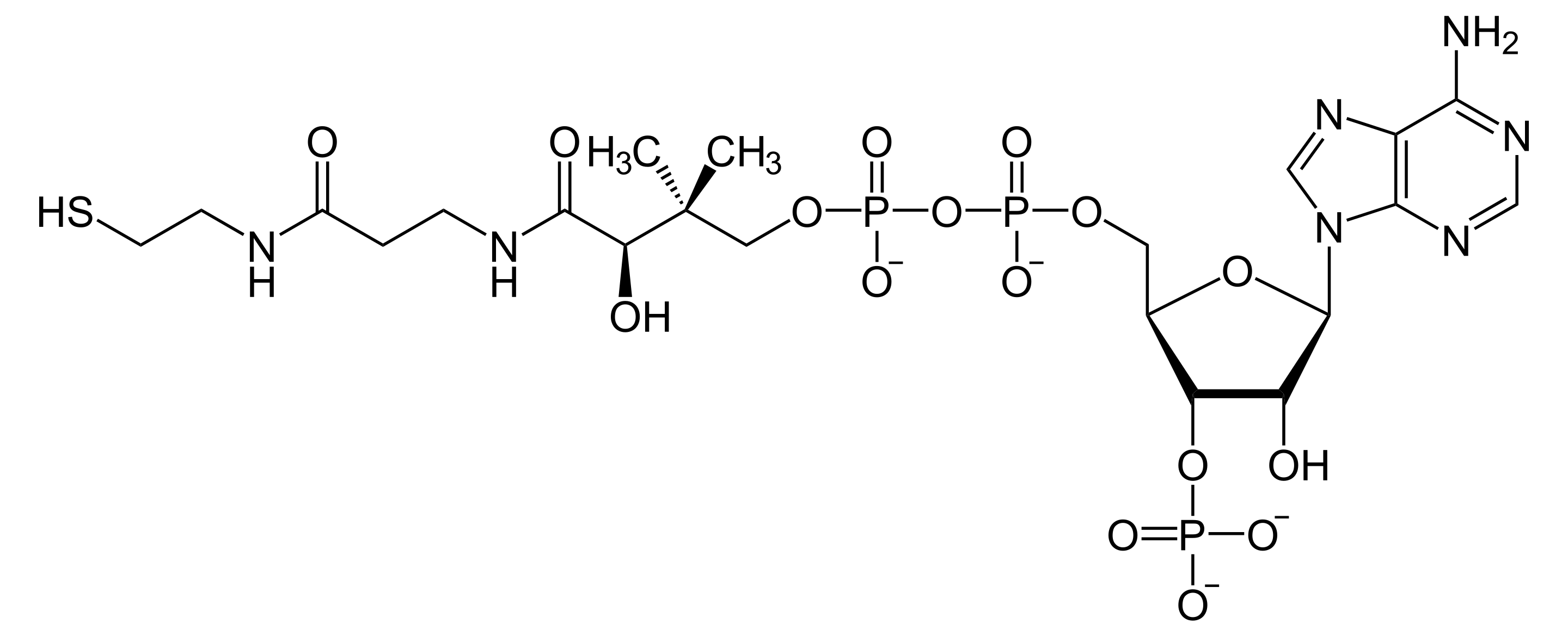
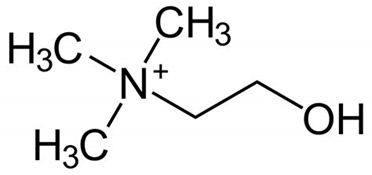
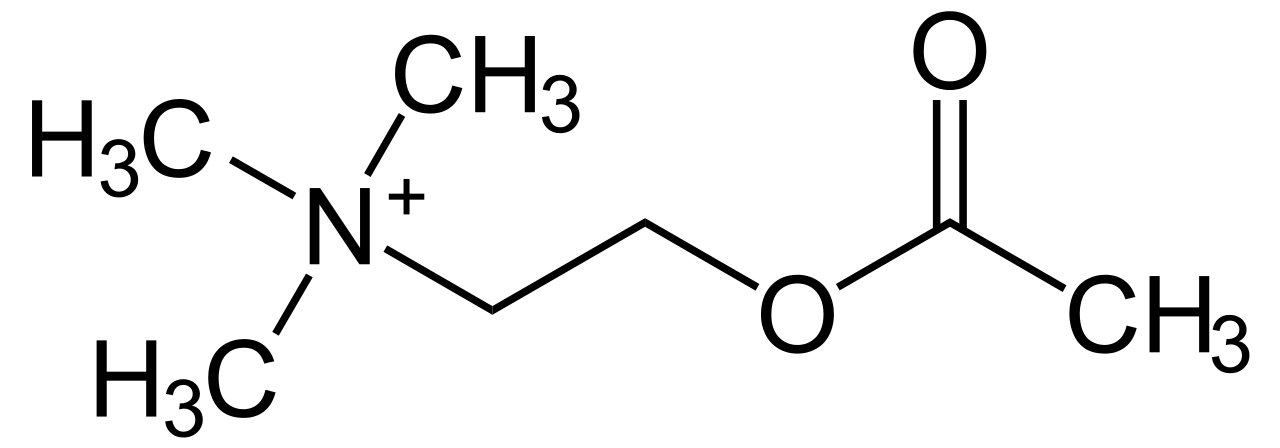
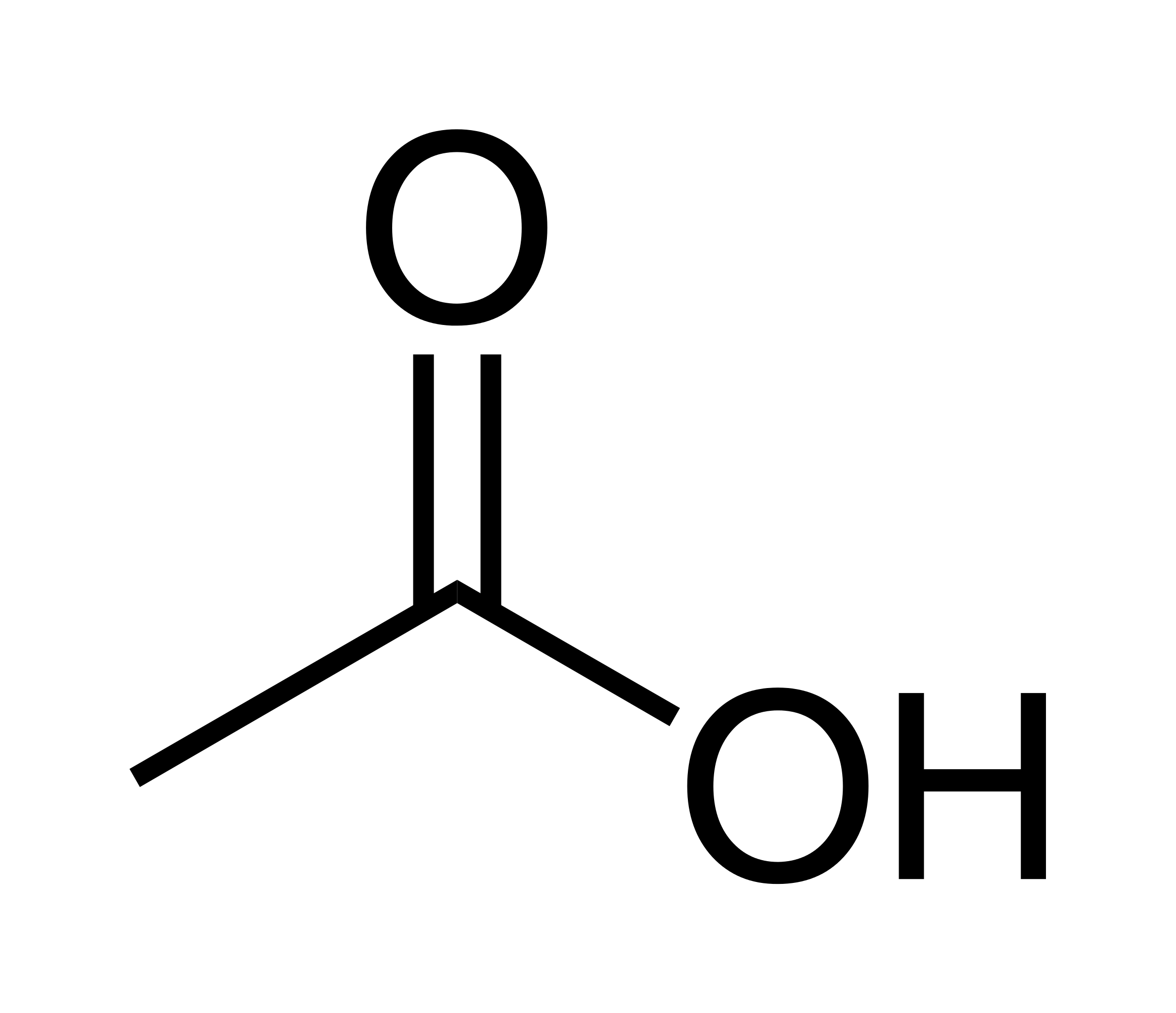
Acetylcholine is produced from choline and acetyl-CoA in specific populations of “cholinergic” neurons by the enzyme choline acetyltransferase, which just transfers the acetyl group. (The presence of choline acetyltransferase is generally the criterion by which a neuron is labelled “cholinergic”). It is broken down by the similarly named acetylcholinesterase into acetic acid and choline. The lifestyle of a typical acetylcholine molecule is as follows:
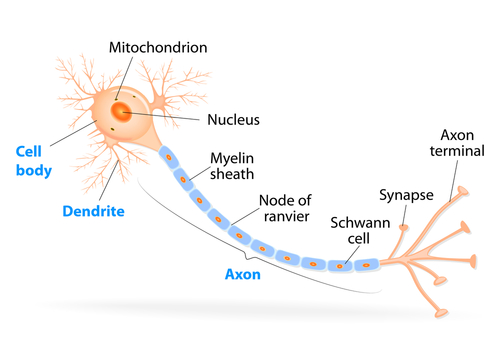
Parts of a neuron. (Source).